Hydrogen is an energy carrier with a high energy density per weight, but it is also a light gas. Our article hydrogen describes this in more detail.
Since hydrogen is such a light gas, the DASH solid-state hydrogen storage systems are a interesting option for the hydrogen infrastructure. In these storages, hydrogen is stored neither in the liquid nor in the gaseous form. Instead a solid and inorganic carrier material captures the hydrogen, the metal hydride.
Basics of the technology #
The basis of this form of hydrogen storage is that the metallic compounds used by GRZ Technologies absorb the hydrogen under the right conditions. This process is shown below.
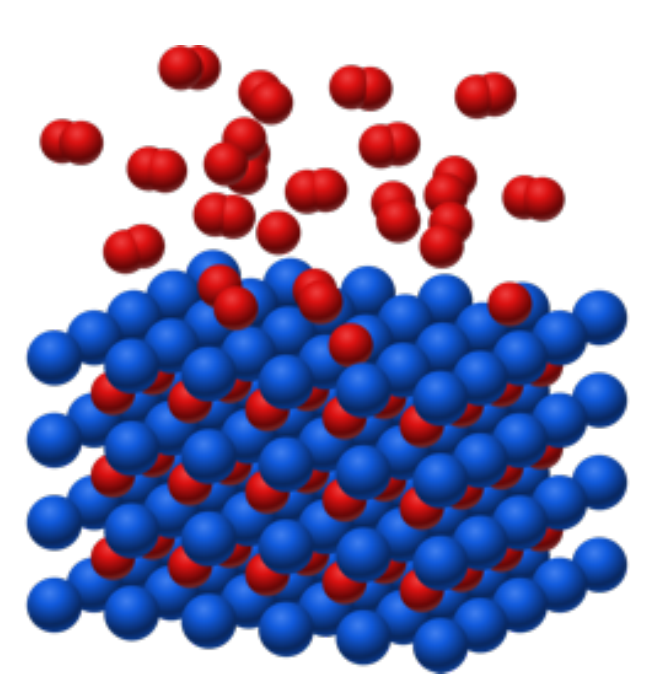
During absorption, the hydrogen molecules (H2) split into individual hydrogen atoms (H). The individual hydrogen atoms then move into the interstitial sites of the metal alloy. The distances between the individual atomic nuclei become significantly smaller than they would be the in the gas phase. As a result, the volumetric density of the hydrogen storage is very high, and so is the energy density of the system. GRZ uses many different alloys for this process, depending on the application. An example of an alloy is LaNi5. The following chemical reaction takes place, when an alloy absorbs hydrogen:
LaNi5 + 3H2 ⟶ LaNi5H6
It is important to state that this alloy is a single example from a whole class of materials. GRZ Technologies engineers develop the best material for each product and thereby optimize the properties of the overall system.
Technical advantages #
However, to use metal hydrides as a solid-state hydrogen storage, not only the selection of the material is decisive. The properties of the overall storage system must be optimized too. We describe this in greater detail in the section “System Design and Manufacturing”.
A key aspect of DASH storage technology is the high volumetric storage density, which depends on the storage material. The volumetric density is limited both by the size of the gaps between the atoms in the carrier material and the distance between the individual H atoms. According to the Westlake criterion (see, e.g., D.G. Westlake, J. Less-Common Metals 91 (1983), pp. 275-292), volumetric storage densities of up to 245 kgH2/m3 are theoretically possible with this technology. For comparison: liquid hydrogen has a density of 71 kgH2/m3 and gaseous hydrogen at 900 bar approx. 40 kgH2/m3. GRZ’s metal hydride-based technology is extremely cycle-proof and allows for lifetimes of 25 years or more. We can use the entire available capacity without restrictions. Finally, an important feature of the technology is its environmental friendliness.
Qualtitative comparison of storage methods #
The metal hydride hydrogen storage device DASH impresses with a significantly reduced ecological footprint compared to existing competing energy storage solutions such as lithium batteries. The storages are fully recyclable, and the energy used for the production of the storages is much lower. The properties of this form of hydrogen storage are summarized and compared to other forms of hydrogen storage in the table below:
| Pressurized gas (low pressure) | Pressurized gas (high pressure) | Liquid | Solid state | |
| Levelized cost of storage | Low | High | High | Low |
| Compressor or liquefier required | No | Yes | Yes | No |
| Volumetric density | Very low | Intermediate to high (700 bar(g)) | High | Very high |
| Energy losses during charging/discharging | No | Yes (compression) | Yes (liquefaction) | No |
| Safety characteristics | Acceptable | Acceptable | Acceptable | Excellent |
| Typical pressure levels | 30 bar(g) | 200-700 bar(g) | Ambient | Ambient to 45 bar(g) |
| Other advantages | • Widely available • Low CAPEX | • Scalable | • Maintenance free | |
| Other disadvantages | • Not the entire storage capacity can be used • Safety-related restrictions and cost | • Not the entire capacity can be used • Safety-related restrictions and cost | • Safety-related restrictions and cost • Technical complexity: boil-off, continuous cooling required, etc. | • Lower gravimetric density |





