A fuel cell is an electrochemical device that converts chemical energy into electricity. It generates a potential by separating the reactions. Hydrogen undergoes oxidation at the anode, while oxygen undergoes reduction at the cathode. Currently, three main types of fuel cells are either under development or in operation. Each type offers unique advantages, limitations, and potential applications. The table below summarizes the key features of these different types of fuel cells:
| Fuel cell type | Operating temperature | Cathode gas | Electrolyte | Anode gas |
| Proton exchange membrane | 50-100 °C | Air (O2 + N2) | Polymer (H+) | H2 |
| Alkaline | 50-100 °C | Air (O2 + N2) | KOH | H2 |
| Solid oxide | 500-1000 °C | Air (O2 + N2) | ZrO2 (O2-) | CH4, CO |
Proton exchange membrane (PEM) fuel cells #
These fuel cells utilize a solid polymer as an electrolyte and feature porous carbon electrodes embedded with a platinum catalyst. They operate using only hydrogen, oxygen from the air, and water, eliminating the need for corrosive fluids that other fuel cells might require. Typically, these fuel cells are powered by pure hydrogen supplied from storage tanks or onboard reformers.
PEM fuel cells function at relatively low temperatures, around 50-100°C. However, the platinum catalyst is highly susceptible to CO poisoning. For this reason, an additional reactor is needed to reduce CO in the fuel gas if the hydrogen originates from alcohol or hydrocarbon fuels. To address this issue, developers are investigating platinum/ruthenium catalysts that exhibit greater resistance to CO.
Transporation and stationary applications often use PEM fuel cells. Structurally, a proton exchange membrane fuel cell comprises a proton exchange membrane (polymer electrolyte) positioned between an anode and a cathode. The figure below provides a schematic representation of a PEM fuel cell.
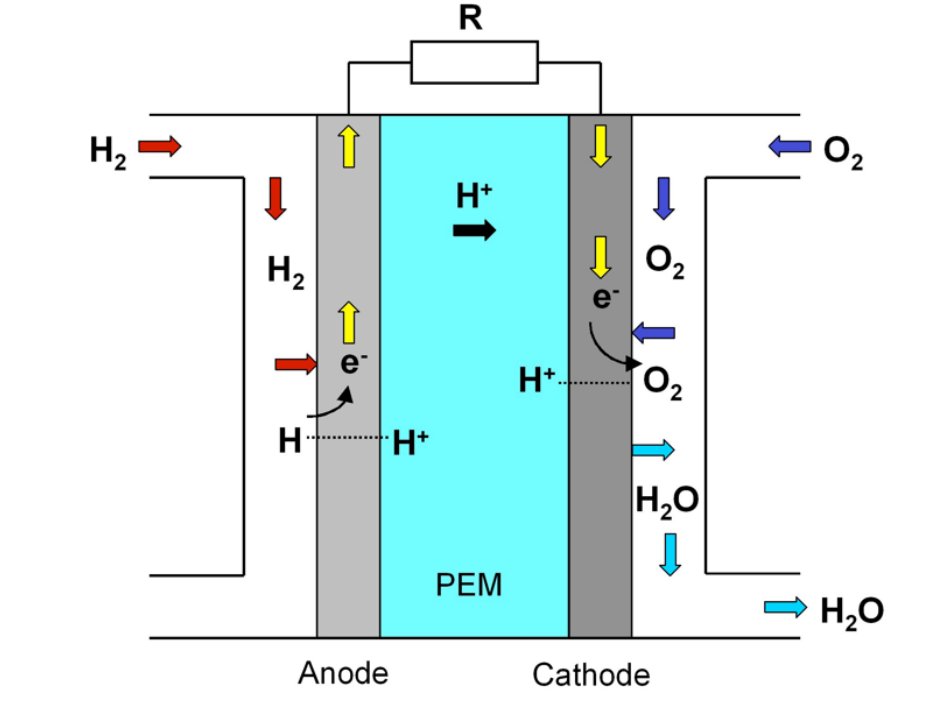
The processes occurring in the fuel cell are as follows:
- Hydrogen fuel introduction: Hydrogen fuel is channeled through flow field plates to the anode on one side of the fuel cell. Oxygen from the air is directed to the cathode on the opposite side.
- Oxygen reaction at the cathode: At the cathode, oxygen reacts with the protons from the proton exchange membrane and the electrons from the electrode.
- Hydrogen dissociation at the anode: On the anode, hydrogen molecules dissociate and adsorb as hydrogen atoms on a platinum catalyst.
- Proton transfer: The proton exchange membrane allows the positively charged hydrogen ions to pass through to the cathode.
The overall reaction occurring in the PEM fuel cell is: 2H2 + O2 → H2O.
Alkaline fuel cells #
They are among the first fuel cell technologies developed. They have been widely used in the U.S. space program to produce electrical energy and water onboard spacecrafts. These fuel cells utilize a potassium hydroxide solution in water as the electrolyte. They can employ various non-precious metals as catalysts at the anode and cathode.
High-temperature AFCs operate at temperatures between 100°C and 250°C. More recent designs function at lower temperatures of approximately 23°C to 70°C. AFCs are high-performance fuel cells due to the rapid rate of chemical reactions within the cell. They are also highly efficient, achieving efficiencies of 60% in space applications.
However, a significant drawback of AFCs is their susceptibility to carbon dioxide (CO2) poisoning. Even small amounts of CO2 in the air (400 ppm) can impair their operation, necessitating the purification of both hydrogen and oxygen used in the cell. This sensitivity to CO2 also affects the cell’s lifespan, or the duration before it needs replacement. Nonetheless, AFC stacks have demonstrated stable operation for more than 8000 hours.
Solid oxide fuel cells (SOFC) #
This type utilizes a hard, non-porous ceramic compound as the electrolyte. Due to the solid nature of the electrolyte, these cells do not need to be constructed in the plate-like configuration typical of other fuel cell types. SOFCs are anticipated to achieve an efficiency of around 50-60% in converting fuel to electricity.
SOFCs operate at high temperatures, approximately 1000°C. This high-temperature operation eliminates the need for precious-metal catalysts. Additionally, SOFCs are the most sulfur-resistant type of fuel cell. They are capable of tolerating significantly higher levels of sulfur compared to other cell types. They are also immune to carbon monoxide (CO) poisoning, allowing CO to be used as a fuel.
However, the high operating temperature presents certain disadvantages. It results in a slow startup and necessitates substantial thermal shielding to retain heat and protect personnel. While this may be acceptable for utility applications, it poses challenges for transportation or small portable applications.
In the past years, proton exchange membrane fuel cells have emerged as the most-used technology for
industrial applications at the large scale. In particular, the automotive industry has focused almost
exclusively on this technology. The DASH Power system manufactured by GRZ Technologies uses PEM fuel cells.





